pISSN : 3058-423X eISSN: 3058-4302
Open Access, Peer-reviewed
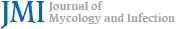
pISSN : 3058-423X eISSN: 3058-4302
Open Access, Peer-reviewed
Minah Cho,Juan Yang,Won Hee Jung,Hei Sung Kim
10.17966/JMI.2024.29.1.7 Epub 2024 March 28
Abstract
A 20-year-old female patient presented with localized erythematous scaly lichenified plaques on her right lower leg. Despite traditional treatments, the lesions consistently recurred in previously affected anatomical locations. Bacterial culture was performed, and Staphylococcus simulans was identified on both lesional and nonlesional skin. Histopathological examination revealed parakeratosis, Munro microabscess, hypogranulosis and regular acanthosis with an elongated rete. Based on clinical and histological findings, the patient was finally diagnosed with possible psoriasis. Although S. simulans is a common livestock colonizer, it rarely colonizes human skin. Herein, we report the first case of S. simulans colonization in a recurrent psoriatic lesion in previously affected anatomical locations.
Keywords
Psoriasis Staphylococcus simulans
Psoriasis is an immune-mediated chronic inflammatory disease characterized by skin symptoms, articular symptoms, and other systemic symptoms. Although the pathogenesis of psoriasis has not been fully elucidated, it is a multifactorial disease driven by both genetic predisposition and exposure to diverse environmental factors. Triggering factors include biomechanical stress, diet, drugs, alcohol, smoking, and in- fection1. Staphylococcus simulans (S. simulans) is a common livestock colonizer and a rare human colonizer. In this report, we present the case of S. simulans colonization in a recurring psoriatic lesion in anatomical areas previously affected.
A 20-year-old female patient presented with localized erythematous scaly lichenified plaques on the right lower leg (Fig. 1A). These lesions first appeared nine months ago and spread as she scratched herself constantly because of severe pruritus. She had no relevant personal or family history, and she also had no history of close contact with animals. The potassium hydroxide (KOH) smear was negative. The lesional and nonlesional skin of the right lower leg was rubbed 20 times (10 times in one direction and 10 times perpendicular to this direction) with a cotton stick for bacterial culture. For molecular identification of bacteria, a single colony was inocu- lated in tryptic soy broth (TSB; 1.7% casein peptone, 0.3% soybean peptone, 0.25% glucose, 0.5% sodium chloride, 0.25% dipotassium phosphate, and 1.5% agar) and cultured at 37℃2. Genomic DNA was extracted following the method described in a previous study3. The 16S rDNA region was identified using the universal primers 27F (5'- GAGTTTGA- TCMTGGCTCAG -3') and 1492R (5'- TACGGYTACCTTGTT- ACGACTT -3')4. The PCR product was sequenced for species identification. The resulting sequence was blasted against the nucleotide sequence database of the National Center for Biotechnology Information. The susceptibility tests were performed using the Muller-Hinton broth (MHB) medium and minimal inhibitory concentrations (MICs) were determined per the Clinical and Laboratory Standards Institute guidelines5. S. simulans was identified on lesional and nonlesional skin. MICs of vancomycin for both isolated strains were 2 μg/ml. MICs of methicillin for both strains were found to be in the 2~4 μg/ml range, indicating that the isolate showed slightly decreased susceptibility to vancomycin and methicillin.
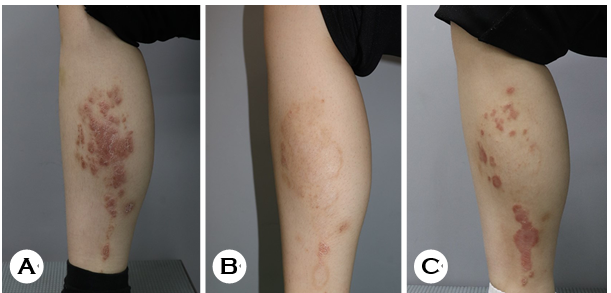
With a clinical impression of lichen simplex chronicus sec- ondary to severe pruritus, we prescribed oral cyclosporine (200 mg/day) and oral antihistamine. After two months of cyclosporine treatment, the lesions improved significantly, leaving postinflammatory hyperpigmentation (Fig. 1B). How- ever, erythematous scaly plaques repeatedly recurred on the same area (Fig. 1C), and punch biopsy was performed. Histopathological evaluation revealed parakeratosis, Munro microabscess, hypogranulosis, and regular acanthosis with an elongated rete (Figs. 2A and 2B). Mild perivascular lympho- cytic infiltration was observed in the dermis. Periodic acid-Schiff (PAS) staining was negative. Per our clinical and histo- logical findings, the patient was finally diagnosed with possible psoriasis.
It has been suggested that infection may play a role in trig- gering and maintaining psoriasis. In particular, the association between streptococcal throat infections and guttate psoriasis is well-established. Additionally, a possible link has been suggested between exacerbations of existing plaque psoriasis and streptococcal infections6. Streptococcal infections can act as both inducing and aggravating factors of psoriasis, possibly by stimulating T-cell proliferation through superantigens and promoting the migration of skin-homing T cells via molecular mimicry between streptococcal and skin-specific epitopes7,8. Among Staphylococcus species, Staphylococcus aureus (S. aureus) infection have been found to be implicated in the disease activity of psoriasis. Its superantigens (particularly staphylococcal enterotoxin-A) play a role in the pathogenesis of psoriasis by stimulating T-cell signaling and interferon-gamma production9. In addition, bacteria such as Helicobacter pylori, Escherichia coli, and Pseudomonas aeruginosa, viruses such as the human immunodeficiency virus and hepatitis virus, and fungi such as Malassezia and Candida may play a role in the pathogenesis and exacerbation of psoriasis10. Further research is needed to determine their exact roles and the mechanisms involved.
To date, there have been no reported associations between S. simulans and psoriasis. S. simulans is a coagulase-negative Staphylococcus that is usually identified in animals such as cattle, sheep, and goats11. However, unlike its common occur- rence in livestock, it rarely colonizes human skin, and there have been only a few cases in which it has acted as a human pathogen causing skin infections11,12, toxic shock syndrome13, osteoarticular infections14,15, native valve endocarditis16, urinary tract infections17,18, and pleural empyema19. On the other hand, S. simulans may function as commensal bacteria, contributing to the maintenance of skin homeostasis rather than acting as a pathogen by producing auto-inducing peptides that block colonization and acute infection by methicillin-resistant S. aureus20. In our case, the use of immunosuppressants such as cyclosporine, along with repeated hand irritation on the lesion, may have affected the skin microbiome or rendered the lesion vulnerable to secondary infection by S. simulans. However, because S. simulans colonization was observed in both lesional and nonlesional skin, it is unlikely to be solely attributable to secondary infection from the external environment. Herein, to the best of our knowledge, we report the first case of S. simulans, a rare human skin colonizer and common livestock colonizer, in a recurrent psoriatic lesion in previously affected anatomical locations. Whether or not the colonization of S. simulans has pathophysiological significance in the pathogenesis and recurrence of psoriasis in this patient remains undetermined. Further research is needed to eluci- date the underlying mechanisms and the significance of the results.
References
1. Yan D, Gudjonsson JE, Le S, Maverakis E, Plazyo O, Ritchlin C, et al. New frontiers in psoriatic disease research, Part I: Genetics, environmental triggers, immunology, pathophysiology, and precision medicine. J Invest Der- matol 2021;141:2112-2122.e3
Google Scholar
2. Doyle JE, Mehrhof WH, Ernst RR. Limitations of thio- glycolate broth as a sterility test medium for materials exposed to gaseous ethylene oxide. Appl Microbiol 1968; 16:1742-1744
Google Scholar
3. Chapaval L, Moon DH, Gomes JE, Duarte FR, Tsai SM. An alternative method for Staphylococcus aureus DNA isolation. Arq Bras Med Vet Zootec 2008;60:299-306
Google Scholar
4. Suzuki MT, Giovannoni SJ. Bias caused by template annealing in the amplification of mixtures of 16S rRNA genes by PCR. Appl Environ Microbiol 1996;62:625-630
Google Scholar
5. Clinical and Laboratory Standards Institute. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 10th ed.; approved standard M07-A10.; Wayne, PA, USA, 2015
6. Gudjonsson JE, Thorarinsson AM, Sigurgeirsson B, Kristinsson KG, Valdimarsson H. Streptococcal throat infections and exacerbation of chronic plaque psoriasis: a prospective study. Br J Dermatol 2003;149:530-534
Google Scholar
7. Valdimarsson H, Thorleifsdottir RH, Sigurdardottir SL, Gudjonsson JE, Johnston A. Psoriasis--as an autoimmune disease caused by molecular mimicry. Trends Immunol 2009;30:494-501
Google Scholar
8. Sigurdardottir SL, Thorleifsdottir RH, Valdimarsson H, Johnston A. The association of sore throat and psoriasis might be explained by histologically distinctive tonsils and increased expression of skin-homing molecules by tonsil T cells. Clin Exp Immunol 2013;174:139-151
Google Scholar
9. Nielsen MB, Odum N, Gerwien J, Svejgaard A, Bendtzen K, Bregentholt S, et al. Staphylococcal enterotoxin-A directly stimulates signal transduction and interferon-gamma production in psoriatic T-cell lines. Tissue Antigens 1998;52:530-538
Google Scholar
10. Zhou S, Yao Z. Roles of Infection in Psoriasis. Int J Mol Sci 2022;23:6955
Google Scholar
11. Shields BE, Tschetter AJ, Wanat KA. Staphylococcus simulans: An emerging cutaneous pathogen. JAAD Case Rep 2016;2:428-429
Google Scholar
12. Tous Romero F, Gutiérrez García-Rodrigo C, Velasco Tamariz V, Llamas Martín R. Acute infection by Staphylo- coccus simulans in the hand of a man. JAMA Dermatol 2016;152:1060
Google Scholar
13. Goda K, Kenzaka T, Hoshijima M, Yachie A, Akita H. Toxic shock syndrome with a cytokine storm caused by Staphylococcus simulans: a case report. BMC Infect Dis 2021;21:19
Google Scholar
14. Désidéri-Vaillant C, Nédelec Y, Guichon JM, Le Louarn S, Noyer V, Sapin-Lory J, et al. Staphylococcus simulans osteitis in a diabetic patient. Diabetes Metab 2011;37: 560-562
Google Scholar
15. Mallet M, Loiez C, Melliez H, Yazdanpanah Y, Senneville E, Lemaire X. Staphylococcus simulans as an authentic pathogenic agent of osteoarticular infections. Infection 2011;39:473-476
Google Scholar
16. Vallianou N, Evangelopoulos A, Makri P, Zacharias G, Stefanitsi P, Karachalios A, et al. Vertebral osteomyelitis and native valve endocarditis due to Staphylococcus simulans: a case report. J Med Case Rep 2008;2:183
Google Scholar
17. Drobeniuc A, Traenkner J, Rebolledo PA, Ghazaryan V, Rouphael N. Staphylococcus simulans: A rare uropatho- gen. IDCases 2021;25:e01202
Google Scholar
18. Shishido M, Kitaoka H, Watanabe K, Fujimoto M, Kumagai T. Acute focal bacterial nephritis caused by Staphylococcus simulans. Cureus 2022;14:e31241
Google Scholar
19. Lal A, Akhtar J, Ullah A, Abraham GM. First case of pleural empyema caused by Staphylococcus simulans: Review of the literature. Case Rep Infect Dis 2018;2018: 7831284
Google Scholar
20. Brown MM, Kwiecinski JM, Cruz LM, Shahbandi A, Todd DA, Cech NB, et al. Novel peptide from commensal Staphylococcus simulans blocks methicillin-resistant Staphylococcus aureus quorum sensing and protects host skin from damage. Antimicrob Agents Chemother 2020;64:e00172-20
Google Scholar
Congratulatory MessageClick here!